
-
Product Development
-
Clinical Trials
-
Quality
-
GxP Audits
-
Regulatory Affairs
-
Pre-authorisation
- Regulatory Roadmap
- Scientific Advice
-
Product information
-
Dossier preparation
- Dossier Gap Analysis
- MA Handling
- Post-authorisation
- Regulatory outsourcing
-
Pre-authorisation
-
Pharmacovigilance
-
QP Service / Importer
Preparation of the clinical part of the registration documentation, clinical review
When submitting a registration application, the Applicant is obliged to attach an assessment of the medicinal product. Such an assessment includes, among other things, a quality assessment and a non-clinical and clinical assessment.
Purpose and scope of clinical documentation
The preparation of the clinical part of the registration documentation primarily involves collecting and critically assessing the available clinical data for the medicinal product being applied for. Data that directly relate to the registration application, i.e., data selected individually for a given medicinal product regarding development strategy, indications, or dosage.
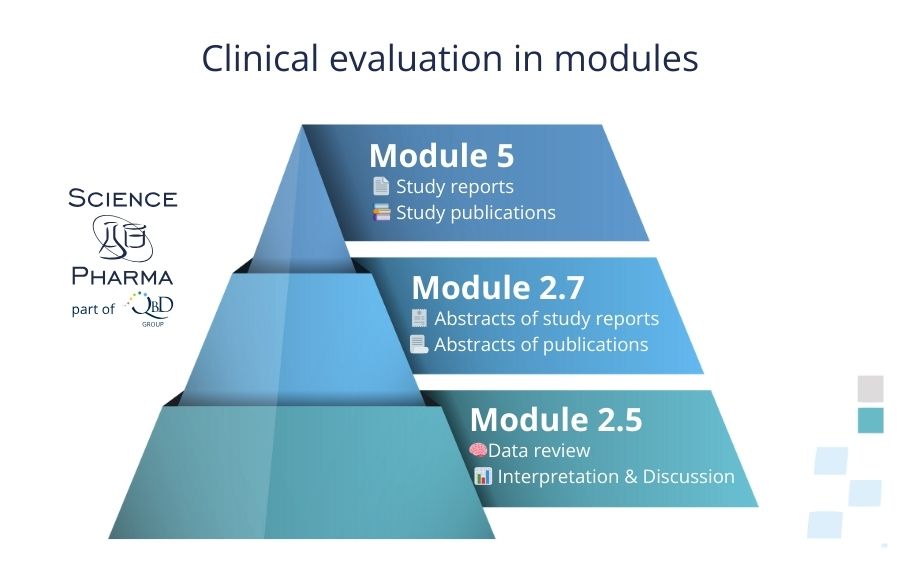
For this purpose, an exhaustive and comprehensive clinical review is performed. The review is summarised with a concise discussion and interpretation of the available data.
A proper clinical evaluation of the medicinal product is crucial for achieving registration success. Of course, the starting point is the Notice to Applicants, NTA.
Clinical evaluation is included in modules: 5 (study reports, study publications), 2.7 (study report abstracts, study publication abstracts) and 2.5 (data review, interpretation and discussion).
The most important elements of a clinical review are:
- description and explanation of the overall clinical development plan for the medicinal product;
- description and assessment of clinical scientific evidence in terms of its quality (including studies methodology, patient selection, studies duration, selection of endpoints, assessment of the control groups, assessment of statistical analysis);
- assessment of the risk-benefit balance based on conclusions from clinical trials, including justification for the proposed indication and dosage;
- analysis of important characteristics of the patient population, including disease stage, risk factors for disease occurrence, age groups, and gender;
- indicating ways to reduce the potential risk to the patient resulting from the use of the product;
- addressing any problems encountered during product development along with how to overcome them, or demonstrating that the problems encountered do not have to constitute an obstacle during the registration of the medicinal product.
Clinical modules in different registration pathways
Following the guidelines in the NTA is not enough, however. The clinical modules will appear slightly differently in various types of registration applications. Here, it is essential to consider several regulations and guidelines specific to the chosen registration strategy and the substances of interest.
In the types of applications based on own research, such as for the original product, the clinical development program of the medicinal product is presented, including ongoing and planned clinical trials. The pillar of this type of application is own research on the efficacy and safety of the developed medicinal product. The results obtained from own research are subject to discussion in relation to literature data and designated treatment standards described in clinical guidelines. Similarities and differences in results between studies or within different patient subgroups are analysed, as well as their impact on the interpretation of data on efficacy.
Scientific evidence from medical information databases may be added to a product’s own clinical data pool. However, it is important that they only constitute a supplement and cannot be the main studies for the product (this is referred to as a so-called mixed application).
In types of applications where reference is made to another medicinal product, such as a generic or hybrid, it is crucial to present a critical analysis of all important issues related to the bioavailability of the product being developed in relation to the reference product. In these types of applications, the comparability of bioavailability between these products translates into the possibility of drawing conclusions about the efficacy and/or safety of the product being introduced to the market. Here, it is crucial to synthetically present the most important elements of the bioequivalence study or prove that the conditions for exemption from performing such a study have been met.
The clinical review for a product with well-established medical use (WEU) is usually based solely on literature data. However, it is crucial that these data fully support the selected indication and dosing regimen in the specified target group and also demonstrate that they are applicable to the product being applied. Some extrapolation is permissible, but many years of experience with Regulatory Authorities are necessary to assess the chances of their acceptance.
Requirements for OTC products – Detailed Safety Assessment
A separate issue is the planned supply status of the developed medicinal product. If the subject of interest is over-the-counter (OTC) drugs, we offer clinical documentation expanded with a detailed safety assessment. Such an assessment aims to demonstrate that the drug can be safely used without medical supervision.

Why us?
We provide comprehensive registration services in Poland and other European countries. We rely on the knowledge and skills of a team of qualified experts. Our effectiveness is proven by numerous registration successes, which you can read about here.
Benefits of cooperation
In accordance with the Applicant’s request, we can prepare comprehensive clinical documentation, including other documentation modules and product information forms. We do not only prepare documentation – it is also possible to commission the entire registration process. All the services you need are in one place!
We usually avoid unnecessary questions from the Regulatory Authorities during the registration process, but if they do appear, we offer support. We will gladly take over the discussion with the Regulatory Authorities and propose solutions!
Are you interested in preparing the clinical part of the registration documentation or in comprehensively registering a medicinal product? Please contact us!

