In today’s rapidly changing pharmaceutical environment, quality is not just a set of procedures – it is the strategic foundation of trust for patients, regulators, and partners.
In this article, we will show how an internal Quality Management System (QMS) can become a powerful driver of continuous safety, regulatory compliance, and effective performance in multiple fields, including the consulting area.
Core Elements of QMS
At the heart of the organization’s QMS lie three essential pillars that ensure its consistency, reliability, and ongoing enhancement.
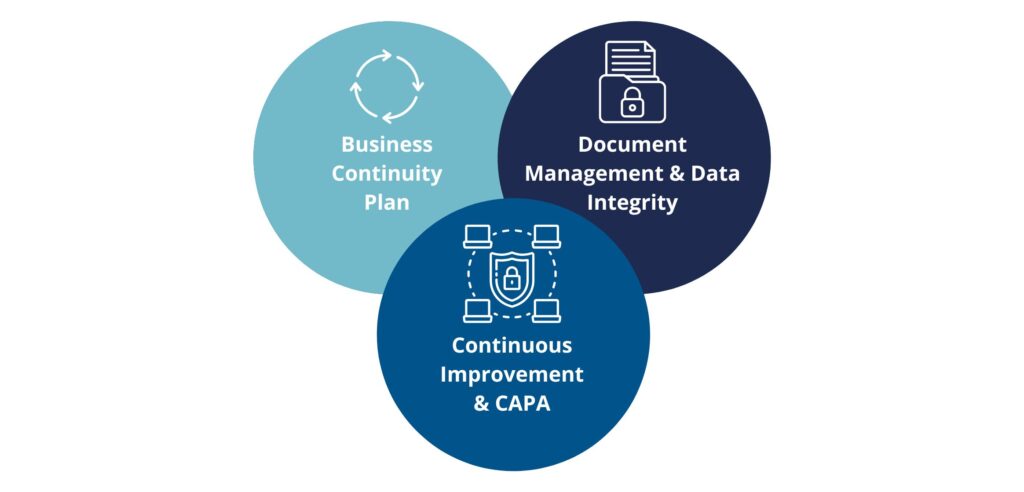
Document Management and Data Integrity
Effective management of documentation and data is the foundation of a reliable QMS.
This includes:
- centralized document repositories,
- clear version control and change history,
- complete audit trails for all updates.
Continuous Improvement and CAPA
A strong QMS must foster a culture of ongoing improvement by proactively identifying issues and implementing systematic corrective and preventive actions (CAPA).
This is achieved through:
- regular review of performance indicators and quality metrics,
- structured root cause analyses leading to effective CAPA implementation,
- knowledge sharing and process optimization to prevent recurrence of nonconformities.
Business Continuity Plan (BCP)
Maintaining quality operations requires preparedness and resilience. A well-defined BCP safeguards the organization against disruptions and maintains compliance even in critical situations.
Key elements include:
- risk assessment and mitigation strategies across core processes,
- clearly defined roles, responsibilities, and communication channels during crises,
- data backup, recovery, and system redundancy to ensure uninterrupted QMS functionality.
Standard Operating Procedures – General
An essential component of an effective QMS is the establishment of general procedures that define and govern the organization’s key processes. These procedures provide a standardized framework ensuring consistency, compliance, and control.
This includes:
- Document and record management – controlling creation, revision, and storage of quality-related documentation,
- Training management – ensuring employees are qualified and competent through planned training activities,
- Risk Analysis – identifying, evaluating, and mitigating potential risks in processes or products,
- Audits – systematic evaluations to verify compliance and identify improvement opportunities,
- Supplier Qualification – assessment and approval of a supplier to ensure compliance with quality and regulatory standards,
- Corrective and Preventive Action – actions designed to correct issues and prevent their recurrence.
Other Processes support by QMS
Beyond general, standardized processes, the QMS also supports other operational, compliance-driven areas, helping maintain quality, efficiency, and process reliability throughout the organization.
An example of this process is the Marketing Authorisation Holder (MAH) service. Thanks to a well-designed standard operating procedure, we efficiently manage all responsibilities involved in transferring MAH obligations to our company.
If you are interested, please visit our website: MAH Service
Another example is eCTD Compilation in CP, DCP, MRP, and national procedures. SciencePharma offers comprehensive support in collecting, structuring, and submitting medicinal product dossiers in the eCTD format, ensuring compliance with all applicable regulatory requirements.
If you are interested, please visit our website: eCTD compilation
The next is Pharmacovigilance, which is the process of monitoring the safety of medicines. We offer our clients the implementation of our pharmacovigilance system covering all quality aspects of the entire system.
If you are interested, please visit our website: Pharmacovigilance
Quality from Planning to Delivery
An effective QMS goes beyond safety-related activities – it also ensures that every customer project and task is managed in accordance with clearly defined procedures.

At SciencePharma, each project is subject to standard procedures that ensure:
- Clear definition of scope and objectives,
- Gap analysis,
- Dedicated leaders and coordinators for each project,
- Controlled communication and documentation,
- Monitoring and review of results.
Strict supervision within the QMS ensures regulatory compliance, operational efficiency, and reliability in every client collaboration.
An effective Quality Management System is fundamental to patient safety and regulatory compliance. Robust quality oversight enables organisations to improve opportunities, maintain compliance, and uphold the highest standards throughout the product lifecycle.
Guided by clearly defined operating procedures, SciencePharma provides efficient support to pharmaceutical companies operating in accordance with regulations.


