Safety data exchange is a crucial domain in the Pharmacovigilance area, ensuring that all safety information is shared effectively and efficiently. Safety data can be understood as detailed information about a chemical/ biological substance or mixture, including its impact on health, properties, threats, risks, and security measures. Timely safety data exchange is the key to fulfilling the legal obligations imposed on the Marketing Authorization Holder (MAH) by pharmaceutical law and local legislation. Regulatory Authorities worldwide expect reporting of safety information, such as ICSRs (Individual Case Safety Reports), to conduct the continuous evaluation of the benefit-risk profile of drugs. To help MAH meet regulatory requirements successfully and fulfill its legal obligations on time, several international guidelines have been developed, e.g., the Good Pharmacovigilance Practice (GVP). To find out more about electronic exchange of safety information in the EU, please visit GVP, section VI.C.6.
Furthermore, the Safety Data Exchange Agreement (SDEA) plays a meaningful role in complying with these regulations, helping entities by establishing clear rules for data exchange.
What’s the essential of Safety Data Exchange Agreement?
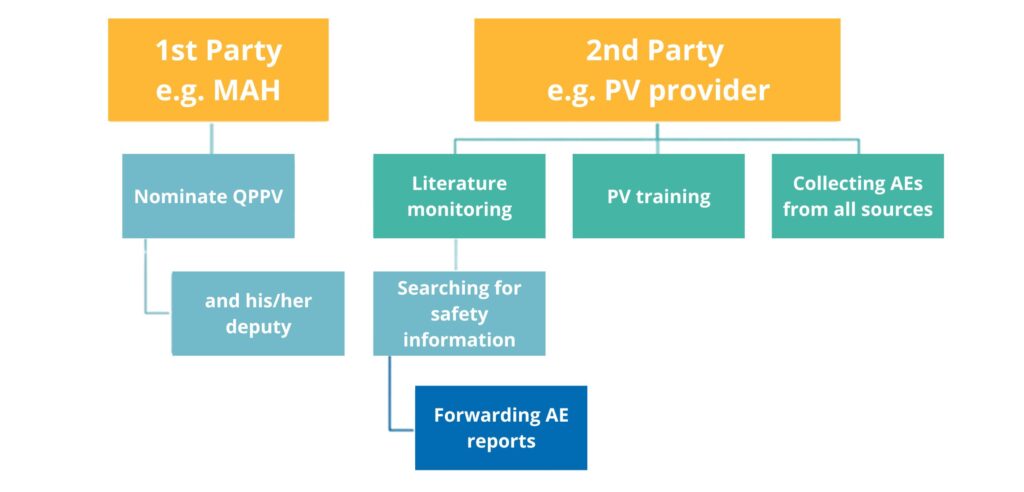
SDEA is a written, legally binding contract between two or more entities involved in the lifecycle of a medicinal product. It defines how, when, and at what frequency safety data will be exchanged between parties. Moreover, SDEA defines the range of responsibilities for each party with respect to the agreed pharmacovigilance activity, and the sample division of responsibilities or activities between SDEA’s parties is presented in Figure 1. According to the Reflection paper on Good Manufacturing Practice and Marketing Authorisation Holders, there is no provision for an MAH to delegate responsibilities to other parties. However, tasks and activities related to those responsibilities may be delegated. All parties within the scope of their responsibilities or activities should actively cooperate to ensure an appropriate vigilance system.
Why SDEAs are so significant?
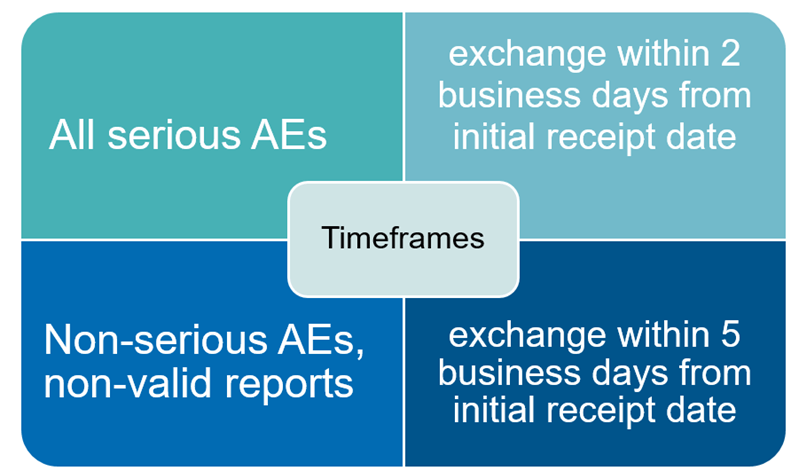
SDEAs are designed to clearly set out each party’s obligations and activities/responsibilities. Appropriate provisions in the contract prevent duplication of PV activities or accidental omission of required activities. Moreover, SDEAs include detailed timeframes for safety data exchange as presented in Figure 2. Clearly described rules of cooperation reduce the risk of non-compliance. Close and efficient cooperation between the parties to the Safety Data Exchange Agreement leads to greater effectiveness in drug safety supervision.
What information should be included in SDEA?
- appoint a Qualified Person Responsible for Pharmacovigilance (QPPV) (and her/his Deputy), who has appropriate qualifications and a thorough understanding of all applicable regulatory requirements in the field of pharmacovigilance
- ensure that QPPV (or her/his Deputy) will be available on a 24-hour basis to ensure fulfillment of pharmacovigilance obligations
- perform local literature searches and transmit the search results to each party
- receive, forward, and handle requests from Competent Authorities
- prepare, implement, and follow the agreed procedures
- receive, forward, and handle AE reports
- conduct follow-up with reporters
- preparation of safety reports such as Periodic safety update reports (PSURs) and Addendum to clinical overview
- preparation and maintenance of Risk Management Plans (RMPs)
- conduct a reconciliation process between the parties
- storage documentation
- perform PV training and establish a way of verifying the gained knowledge
- conduct mutual audits
- receive, forward, and handle product complaints and/or medical enquiries, regardless of whether the event has been or has not been related to an adverse event report/other safety information
Who can become a Party of SDEA?
The scope of activities described in the SDEA depends on the partners with whom the SDEA is signed. On the one hand, the scope of the SDEA can be wide, as in the case of an agreement between MAH and a PV provider. On the other hand, the range of activities can be narrow, for instance, in an agreement between MAH and a manufacturer, where the scope of the SDEA is usually limited to the manufacturer forwarding AE notifications to MAH within the appropriate timelines.
Every entity involved in the pharmacovigilance during the lifecycle of a medicinal product can be a party to SDEA, especially:
- Marketing Authorization Holder
- Importer
- Manufacturer
- Distributor
- Contract Research Organization (CRO)
- Co-marketing or co-distribution Partner
- License Holder
What about EU and non-EU cooperation?
In times of globalization, European (EU) MAHs are increasingly launching products to non-EU markets – e.g. Ukraine, Belarus, Kosovo, the United Arab Emirates, but it is worth remembering that the group of non-EU countries also includes the United Kingdom after Brexit (for more information about Brexit in relation to EU MAHs please find here.
To ensure that EU MAH will be able to fulfil its pharmacovigilance responsibilities in different marketed territories inside and outside the Europe, it can make a contract with non-EU company which shall provide needed support. EU MAH can decide to appoint non-EU company for local pharmacovigilance activities on MAH’s behalf. To act in accordance with GVP Module VI section VI.C.2.2., each marketing authorisation holder shall have in place a system for the collection and recording of all reports of suspected adverse reactions in the EU or in third countries which are brought to its attention (…). All parties of the agreement shall cooperate to ensure appropriate vigilance system in accordance to legal obligation imposed on MAH by EU pharmaceutical law and local legislation on non-EU territory as presented in Figure 3. It should be emphasize, that all AR/ADR reports and other safety information collected from non-EU territory need to be provided to EU MAH. Also, according to GVP Module VI, section VI. App.3.2. all serious ICSRs need to be submitted to EudraVigilance database within 15 days.

Who’s an expert in pharmacovigilance activities resulting from SDEAs?
Experts from the Pharmacovigilance Department of SciencePharma have extensive experience in creating a Safety Data Exchange Agreement. As a Party of SDEA, SciencePharma makes every effort to fulfil all contractual obligations on time. MAH, which decides to delegate local pharmacovigilance activities to SciencePharma, can be sure of the highest quality services. For more details, please visit our pharmacovigilance services.


