
-
Product Development
-
Clinical Trials
-
Quality
-
GxP Audits
-
Regulatory Affairs
-
Pre-authorisation
- Regulatory Roadmap
- Scientific Advice
-
Product information
-
Dossier preparation
- Dossier Gap Analysis
- MA Handling
- Post-authorisation
- Regulatory outsourcing
-
Pre-authorisation
-
Pharmacovigilance
-
QP Service / Importer
Promotional materials review
Promoting medicinal products in Poland is a complex task. It demands not only creativity but, most importantly, strict adherence to current regulations. As a consulting company, SciencePharma has a deep understanding of these challenges. We offer comprehensive support in verifying promotional materials, ensuring their legal compliance and effectiveness in reaching the right audience.
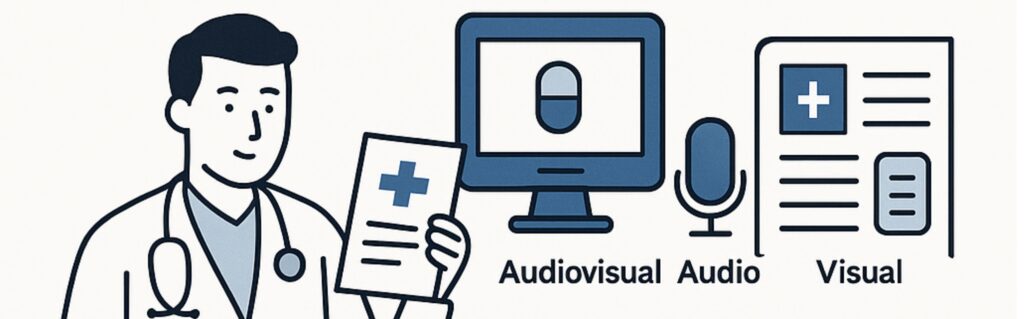
What are promotional materials for medicinal products?
Promotional materials of medicinal products are various forms of promotion aimed at informing people about a medicine with a marketing authorisation or encouraging its use. The purpose of promotional materials is to increase the number of prescriptions, sales, supply or consumption of a product.
Promotional materials may be addressed to the general public as well as to the persons qualified to prescribe or trade medicinal products. They may take various forms, including:
- Audiovisual
- Audio
- Visual
Why is it important to review promotional materials?
All promotional materials presented or distributed in Poland must comply with the legal criteria. Under the provisions of the Pharmaceutical Law, marketing authorisation holders are responsible for ensuring that advertising complies with the applicable regulations. Ignoring these regulations can lead to serious legal and reputational consequences. Additionally, promotional materials should be prepared in a way that is understandable and adapted to the target recipients. Only then can they effectively reach the dedicated target audience and help achieve the intended goals, such as increasing sales or improving product awareness.

A vague or misleading message not only violates regulations but also wastes promotional resources.
How do we review promotional materials at SciencePharma?
At SciencePharma, we approach the verification of promotional materials with the utmost diligence and precision. Every received material intended for use in Poland is assessed by our Experts for compliance with the provisions of key legal acts:
- The Pharmaceutical Law Act of September 6, 2001, which forms the legal basis regulating aspects of medicinal products in Poland, including their advertising.
- The Minister of Health’s guidelines on the advertising of medicinal products, as set out in the Regulation of November 21, 2008, which contains details on the content, form, and principles of disseminating drug advertisements.
Our analysis covers not only the substantive content but also the visual aspects of the presented materials. We evaluate the clarity of the message, readability, aesthetics, and whether the visual form supports, rather than hinders, the understanding of key information. This approach enables compliance of advertising materials with legal regulations, while also considering their effectiveness and professional quality.
Trust SciencePharma and promote your product responsibly
Since 2004, SciencePharma has been a leader in the consulting industry, supporting Polish and foreign clients with their projects. SciencePharma is comprised of a diverse team of professionals from a range of fields, all of whom are dedicated to providing the highest quality service and ensuring customer satisfaction.

On the basis of their many years of experience, knowledge and, above all, the current guidelines, our Experts thoroughly review the promotional materials provided and suggest other and/or additional solutions to bring the materials into line with prevailing standards or to improve their readability. With extensive experience, our team of experts provides support in developing advertising materials that are both legally compliant and marketing-effective.
If you’re interested in the topic of advertising medicinal products, we invite you to read our blog article on the subject: Medicinal products advertisement – what is it and what requirements must it fulfill?
What do you gain by cooperating with SciencePharma?

Legal Certainty and Penalty Avoidance
Our experts will thoroughly verify your advertising materials for compliance with the current Pharmaceutical Law and the Minister of Health’s Regulation. You will receive detailed suggestions for changes that may help ensure full compliance with regulations, protecting your company from the risk of sanctions and reputational damage.

Increased Communication Effectiveness
We will assess whether your materials are clear and appropriately tailored to the target audience. Based on our analysis, we will provide suggestions for changes that may help make the message clearer, more precise, and more effective in reaching its intended recipients. This may contribute to an increase in prescription volume, higher sales, and improved business performance.
Comprehensive Expert Support
Our versatile team of professionals offers comprehensive support at every stage of your projects. We address questions and clarify doubts, providing solutions tailored to your individual needs.
See how we can support you
Establishing cooperation with SciencePharma is a key step towards the effective implementation of both business and regulatory objectives. Thanks to the broad expertise of our specialists, we flexibly adapt the scope of our services to the nature of your activities. We provide substantive support throughout the entire process.
In the area of advertising materials review, we offer a thorough analysis of their compliance with the applicable legal regulations in Poland, as well as an assessment of the clarity of the message. Based on the submitted materials, we provide detailed comments and suggestions for changes.
If you require assistance in this area, please complete the form below to contact us.

