Importing medicinal products into the European Union is not merely a matter of logistics and trade. It is, above all, an activity strictly regulated by pharmaceutical law, in which quality, safety, and compliance with Good Manufacturing Practice (GMP) requirements are key.
By definition, import also includes storage, quality control during batch release, and distribution. One of the most important elements of this entire process is the importer’s warehouse—a facility with a unique regulatory status that is often mistakenly confused with that of a pharmaceutical wholesaler.
What Is the Role and Specific Function of an Importer’s Warehouse?
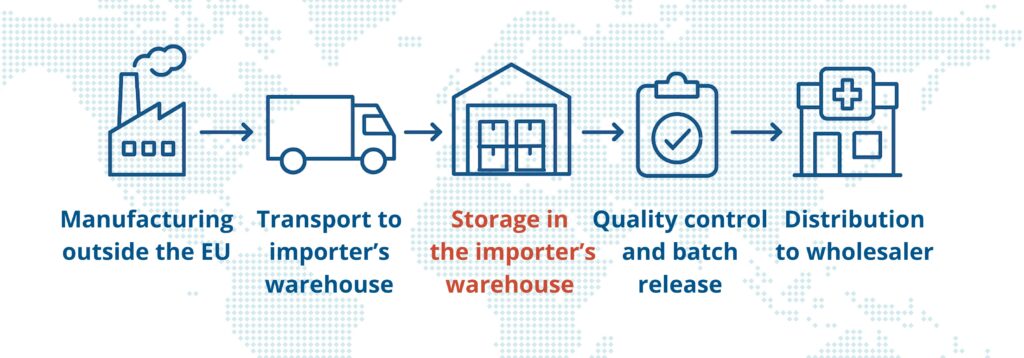
An importer’s warehouse is not just a storage space – it is an integral part of the import process, a critical point in the supply chain that directly affects the availability and quality of medicinal products on the market. It must meet strict quality system requirements in compliance with GMP guidelines, and its operation requires specific authorization.
This is a specialized facility designed to receive and store medicinal products imported from outside the European Union, before they are released for circulation on the EU market.
Products stored in the importer’s warehouse are not yet eligible for sale – they require specific quality checks, including the formal release of each batch by a Qualified Person (QP), as required by GMP.
This is one of the key differences from pharmaceutical wholesaler. Once released by the QP, the products may be distributed to pharmaceutical wholesalers, hospitals, and pharmacies.
What kind of Authorization is required to operate an Importer’s Warehouse?
To operate an importer’s warehouse, a company must obtain an import authorization for medicinal products (MIA – Manufacturer/Importer Authorization) in the scope of the site of physical importation, issued by the national competent authority responsible for pharmaceutical oversight (in Poland – Chief Pharmaceutical Inspectorate).
This authorization grants the right to:
- import medicinal products from third countries,
- store products prior to their release onto the market,
- cooperate with a Qualified Person in the process of batch release
This import license is distinct from a wholesale distribution authorisation and requires compliance with GMP standards, rather than solely with Good Distribution Practice (GDP).
GMP Requirements for an Importer’s Warehouse – What Must Be Met?
1. A GMP-Compliant Quality System
An importer’s warehouse must implement and maintain a GMP-compliant quality system, which is a fundamental difference compared to a pharmaceutical wholesaler. Ensuring GMP compliance guarantees imported medicinal products:
- meet the quality standards specified in the registration documentation,
- are effective and safe for end users – the patients,
- undergo proper quality evaluation before entering the distribution chain.
Operating an importer’s warehouse in compliance with GMP includes, among others:
- providing proper storage conditions (dedicated technical infrastructure, defined zones, monitoring systems, alarms, and access control),
- continuous monitoring of environmental conditions (temperature, humidity),
- developing and implementing detailed Standard Operating Procedures (SOPs),
- validation and qualification of equipment and premises,
- personnel training aligned with roles and responsibilities,
- complete documentation for each batch.
2. Employing a Qualified Person (QP)
Each batch of a medicinal product must be formally released for distribution by a Qualified Person (QP), who confirms the product’s compliance with the registration documentation and verifies that it meets EU requirements, including GMP requirements. The QP plays a crucial role within the importers’ organization, being responsible for oversight and assurance that all product-related activities are conducted in accordance with legal regulations and good practices.
This is achieved through, among other tasks, organizing regular audits, including control of manufacturing sites.
3. Importer’s Warehouse and Outsourcing
It’s important to note that an importer may outsource storage and batch release activities to an external entity—a contract importer —provided that entity holds the appropriate import authorization and GMP-compliant infrastructure. Such cooperation must be formalized in a quality agreement between the parties.
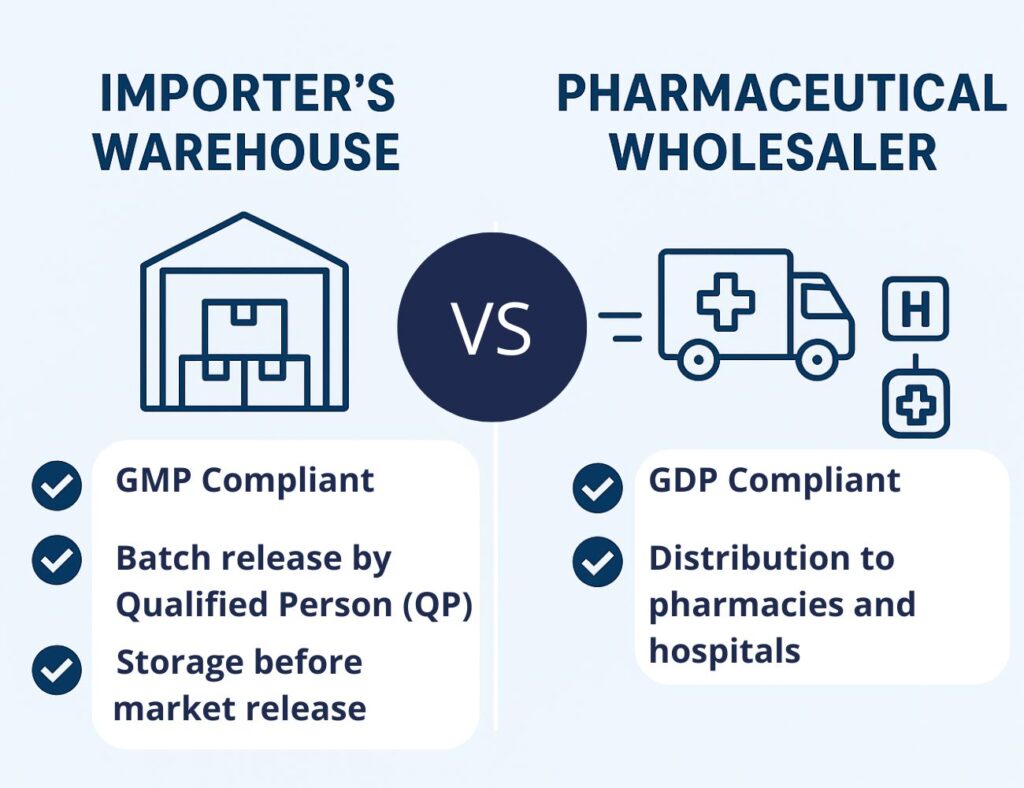
4. How Does an Importer’s Warehouse Differ from a Pharmaceutical Wholesaler – in brief?
While both facilities are involved in handling medicinal products, they differ significantly in their function, regulatory framework, and scope of responsibility:
| Required Authorization | Manufacturer/Importer Authorization | Wholesale Distribution Authorization |
| Regulatory Compliance | GMP – Good Manufacturing Practice | GDP – Good Distribution Practice |
| Qualified Person (QP) | Required | Not required |
| Batch Release Responsibility | Mandatory before market release | Products already released for sale |
| Function | Storage prior to market release | Distribution to pharmacies, hospitals, and other healthcare providers |
| Product eligible for sale | No (before batch release) | Products can be sold and distributed immediately |
To Sum up
An importer’s warehouse is not just a physical space – it is a complex system designed to ensure that each medicinal product entering the EU market meets the highest quality standards.
The differences compared to a pharmaceutical wholesaler are fundamental and relate to key aspects such as legal regulations, the involvement of a Qualified Person (QP), and the obligation to comply with GMP requirements.
Therefore, engaging in import activities – including the setup and operation of an importer’s warehouse – requires expert knowledge of pharmaceutical law and GMP.
Remember – compliance with regulations is not just a formal obligation, but the very foundation of your responsibility to the patient. You can read more about how an importer ensures product safety management in our article.


