Blog

Getting a Handle on the Pharma World: Why Market Research and Planning Are Key
16 kwietnia 2025
Getting a Handle on the Pharma World: Why Market Research and Planning Are Key Let’s face it: the pharmaceutical industry is a constantly shifting landscape. If you want to make a real impact and succeed, just having great drugs and following the rules isn’t enough. You’ve got to get the market and have a smart marketing plan in place. When you have solid market insights, […]

World Backup Day – Regulatory Requirements and Best Practices
20 marca 2025
On March 31st, we celebrate World Backup Day. It’s a perfect opportunity to remind you about one of the key issues in data security, particularly in the pharmaceutical industry. With the ongoing digital transformation that enables more effective management of documentation and processes, data protection becomes a crucial element in ensuring regulatory compliance and business continuity. Why does your company need backups? Compliance with regulatory […]
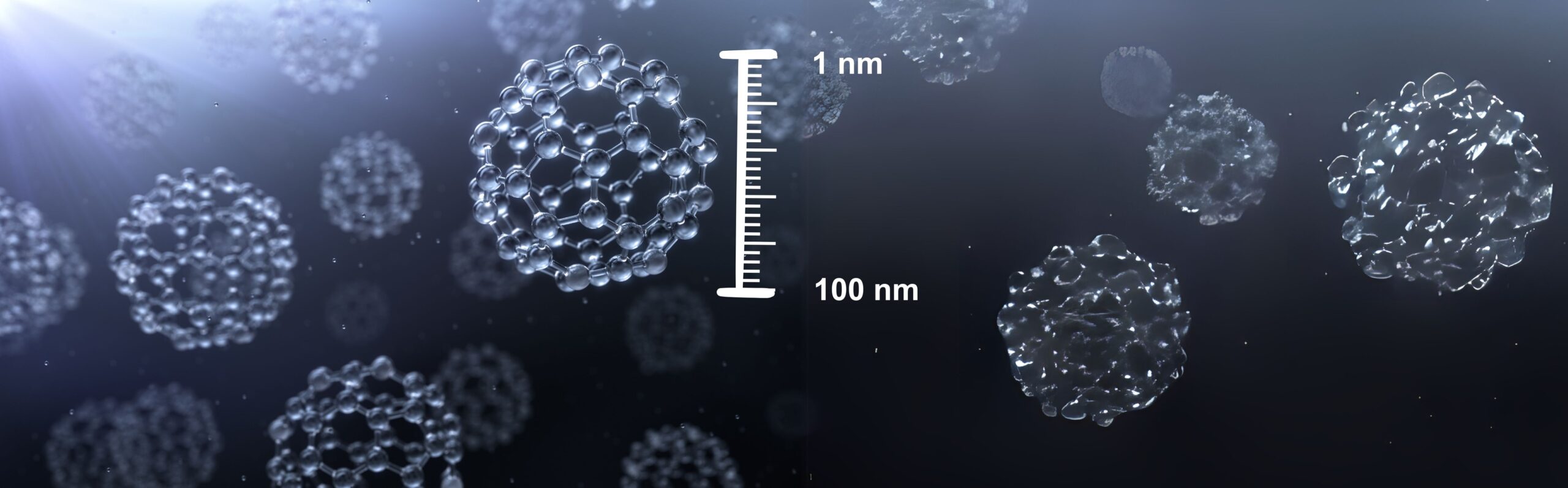
Exploring the potential of Nanoparticles and Nanotechnology systems in medicinal products development and registration
18 lutego 2025
Have any of you used them? Had a contact with them? Nanoparticles are everywhere around us. Undetectable by the human eye, nanoparticles can exhibit significantly different physical and chemical properties to their larger counterparts. Researchers realized the importance of nanoparticles when they found that size can significantly influence the physiochemical properties of such substances. Although, currently, there is a coexistence of different definitions of nanomaterials […]

AI-Enabled Medical Devices – EU’s New Regulatory Landscape
13 stycznia 2025
The Growing Role of AI in Digital Health Artificial Intelligence has evolved significantly since Christopher Strachey developed the first AI program in 1951 (Alowais 2023). In its early stages, AI was mainly a subject of academic study. The field took a major step forward in 1956 when John McCarthy hosted the Dartmouth Conference and introduced the term „Artificial Intelligence”. Today, AI is defined as the […]

What’s the deal with vitamin D?
18 listopada 2024
What is vitamin D? Vitamin D is a series of organic chemical compounds, the most important of which are cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2). They differ chemically only in their side-chain structures, but both play the same role in the body. Both vitamin D3 and D2 are inactive, and only in the body are they transformed into biologically active forms: calcitriol […]

Regulations for psychotropic medications in Poland
09 października 2024
According to Article 41 of Polish Act of 29 July 2005 on Counteracting Drug Addiction, retail trade in psychotropic substances and precursors that are medicinal products shall be conducted by pharmacies, ensuring appropriate storage conditions preventing unauthorised access to these drugs and substances. Additionally, it should be noted that medications containing psychotropic substances shall be dispensed from the pharmacy only on the basis of a specially labelled prescription or requisition […]
Pharmaceutical forms of medicines: from classic to modern solutions
20 września 2024
World Pharmacist Day provides an excellent opportunity to explore the variety of pharmaceutical forms of medicinal products that play a key role in the daily lives of millions of patients around the world. Modern pharmacy is not limited to pills and syrups – it also encompasses modern technologies and innovative solutions that are transforming the landscape of treatment forms. The choice of whether a drug is enclosed in a capsule or administered in liquid […]

Supply chain optimization for medicinal product safety
22 sierpnia 2024
In the summer period, as temperatures rise, ensuring the safety of medicinal products becomes important. High temperatures may significantly affect product quality, leading to serious consequences for patient safety and regulatory compliance. Therefore, the optimization of the cold chain supply is a key element of the strategy for every pharmaceutical company. This article discusses the importance of cold chain management in the pharmaceutical supply chain, […]

Buzzing Mutual Benefits: The vital role of bees in medicine and novel veterinary medicinal products to protect honeybees
25 lipca 2024
August is a month, in which in Poland we celebrate the Great Bees Day. It is a suitable occasion to provoke us to think about how much we owe bees and what the world could look like without them. We can spill the beans and admit that humans cannot survive without these tiny creatures. Fortunately, we have some options to protect them and humans from disaster. Let’s look at what bees […]

Sunny Solutions: Unmasking the UV Filters
19 lipca 2024
Sunlight has several beneficial effects in human, such as the production of vitamin D, and induction of β-endorphin expression, which improve well-being. However, excessive sunlight exposure is associated with photo-induced skin damage, namely solar sunburn, hyperpigmentation, photoaging, and the promotion of skin cancers, such as basal cell carcinoma and malignant melanoma. The factor responsible for all the above-mentioned effects (both good and bad) […]

From Idea to Market: Navigating the Path of Medical Device Development
17 maja 2024
The medical device sector grows rapidly and, according to Medical Device Regulation (EU) 2017/745 (hereinafter: MDR), covers a very wide group of products – from simple devices to high-technology machines using artificial intelligence. Regardless of the product complexity, MDR emphasises issues related to the quality, safety and performance of each medical device. Therefore, manufacturers face huge challenges: from end user`s expectations to safety, […]

Is an uncertain remedy better than none? Exploring the usage of off-label drugs and ensuring treatment in Pregnant and Breastfeeding Women
07 maja 2024
A Latin sentence says: anceps remedium melius quam nullum – an uncertain medicine is better than none. Have you ever wondered why the use of drugs in some populations of patients (such as pregnant women or children) is especially encouraged to be reported? The answer to this question is straightforward – it is impossible to carry out a clinical trial in every population of patients. Due to this, we do not have enough data to introduce indications in the […]

