Nowadays in the pharmaceutical industry the rules of Good Manufacturing Practice are something obvious. Quality manufacturing systems and the safety of the medicines are a priority for drug product manufacturers. However, it should be stated that the beginnings of GMP were developed on a number of tragedies.
How the FDA pioneered the development of GMP?
During the XX century, when the first medicines were released to the market the pharmaceutical industry started to develop dynamically. Initially, although it is hard to believe, there was no scrutiny of how medicines were produced. No attention was paid to whether the process was repeatable, safe, or whether the produced medicine was of appropriate quality. In 1937 a company manufacturing one of the first wonder drugs against infection (sulfanilamide) dissolved it in a poisonous liquid. More than 100 people, mostly children, died before the problem was discovered (1).
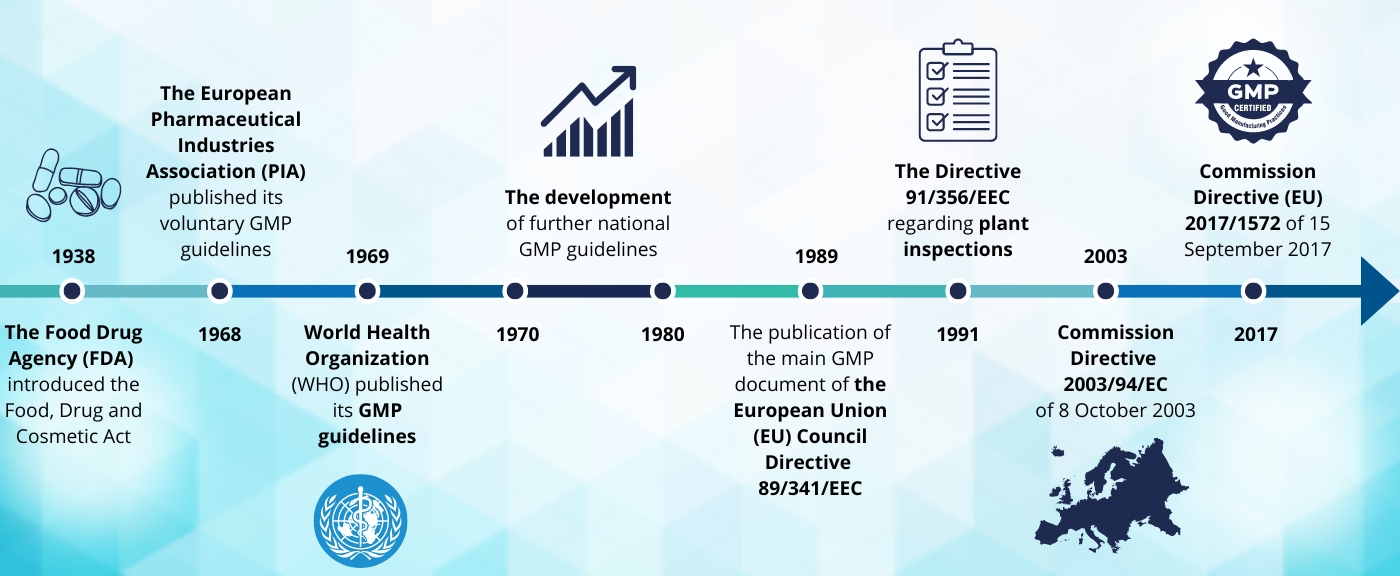
In response to this tragedy, in 1938, the Food Drug Agency (FDA) introduced the Food, Drug and Cosmetic Act, which established registration controls and a factory inspection system (2). Introducing the first regulations (Food Drug and Cosmetic Act) in the US was not sufficient to avoid further tragedies. In 1941 after taking sulfathiazole tablets tainted with the sedative phenobarbital, nearly 300 people were killed or injured. This incident prompted the FDA to revise manufacturing and quality controls drastically, the beginning of what would later be called GMP (3).
During World War II, the batch certification by the FDA became a requirement for certain drugs. Companies have been required to provide samples from each batch to the FDA for testing. Upon successful test results, the agency would give permission for their release. This practice was first applied for the release of insulin in 1941 and for penicillin in 1945. Later drug testing by the FDA was expanded to include all antibiotics. In 1983 such controls were deemed no longer needed and abolished (3).
Meanwhile in Europe
While the FDA was improving regulations aimed at bringing safer drugs to the market, the most tragic accident in the history of pharmaceuticals occurred in Europe. In the 1960s thalidomide was launched in Europe leading to the estimated 10,000 cases of infant deformities (1). This was a trigger for the first initiative taken by the European Pharmaceutical Industries Association (PIA), which published its voluntary GMP guidelines in 1968. Shortly after the World Health Organization (WHO) published its GMP guidelines, which provided a basic outline of GMPs for worldwide use.
The early 1970s and 1980s saw the development of further national GMP guidelines, culminating in the publication of the main GMP document of the European Union (EU) Council Directive 89/341/EEC of May 3, 1989. This Directive requires all EU Member States to comply uniformly with GMP standards during the industrial production of drug products and thus serves as the basis for two separate sets of GMP guidelines issued by the Commission.
The Directive has been repealed by the Commission Directive 2003/94/EC and then replaced by the currently in force Commission Directive 2017/1572. Finally, the European Economic Community (EEC) published the GMP guidelines contained in Volume IV of the “Rules Governing Medicinal Products in the European Community”.
Plant inspections – the important part of GMP.
Plant inspections are one of the most important parts of the GMP. The obligation to carry out inspections at manufacturing sites was formulated firstly in the US in the Food Drug and Cosmetic Act in 1938. In the EU, Directive 91/356/EEC of 13 June 1991 requires that Member States perform repeated inspections to ensure that manufacturers respect the principles and guidelines of Good Manufacturing Practice. After a positive inspection, a GMP Certificate is awarded as proof that the company has good quality standards and implements GMP principles.
As shown in the article, the history of GMP had its dark sides. Fortunately, current regulations allow us to avoid the tragedies that occurred in the past. Currently, the pharmaceutical industry is one of the most restrictively controlled manufacturing industries. Implementing a GMP system in your factory can be difficult and complicated due to the number of standards that must be taken into account when designing your own system to help in the daily operation of the manufacturing site.
SciencePharma has extensive experience in preparing and implementing GMP systems. If you need help in this area, please check out our website. If this article sparked your interest, be sure to look into the begginings of pharmacovigilance for further insights.
Sources:
- Office of Women’s Health, FDA Milestones in Women’s Health: Looking Back as We Move into the New Millennium (FDA, Rockville, MD, 2000), https://webharvest.gov/peth04/20041118000645/http:/www.fda.gov/womens/milesbro.html
- R. S. Heir, C. Chem, MRSC, FIQA, Good Manufacturing Practice: an historical overview and actual status, Drug Information Journal, Vol 28, pp. 957-963, 1994.
- FDA, Milestones of Drug Regulation in the United States https://www.fda.gov/media/109482/download
- https://picscheme.org/en/members retrieved on 11.01.2024


